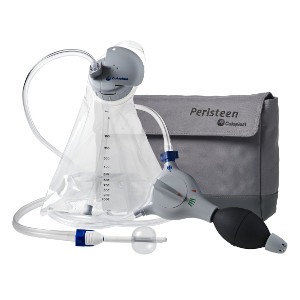
Peristeen for predictable bowel management
PCG Medical is excited to announce that we are the first supplier in the state of Louisiana to offer the Coloplast Peristeen Product for Predictable Bowel Management.
PCG Medical is a leader in providing the best product solutions for catheters and bowel management products in the Gulf Coast region. PCG Medical partners for the best outcomes for each individual customer by working closely with our manufacturing partners to deliver the most innovative and cutting edge solutions.
In May, PCG Medical successfully received the first private insurance prior authorization for Peristeen in the state of Louisiana for a BCBSLA patient. The prior authorization process only took us one day which impressed the Peristeen team at Coloplast as sometimes authorizations can take up to 4-6 weeks.
The next step in getting the Peristeen product to our patients here in Louisiana is to have our healthcare providers trained so that they can properly and safely teach patients how to use the Peristeen product.
Therefore, PCG Medical is working closely with Coloplast to have the urology and rehab departments at Children’s Hospital of New Orleans formally trained by the Coloplast Peristeen nurse trainer. The Peristeen training for the urology staff at Children’s Hospital is scheduled for Thursday June 26..
What is Peristeen and how does it work?
Peristeen is a transanal irrigation system for managing bowel dysfunction and is indicated for use by SCI, Spina Bifida, MS or other neurogenic disorders. Coloplast has experienced great success especially among users who have been struggling with their bowel program for an extended time.
Peristeen enables people suffering from severe constipation and fecal incontinence to improve their quality of life and can significantly reduce the time spent on their bowel program as compared with conservative bowel management solutions.
Peristeen was introduced in Europe in 2004. Since then more than 15,000 patients are using this device and it is making a significant difference in their quality of life. The FDA approved Peristeen for use in the US in August 2012.
Check out the Peristeen video instruction to learn more. This link is easily found through PCG Medical’s web site video-portal page at http://www.pcgmed.com/peristeen
Reimbursement:
Currently there is no national reimbursement code (HCPCS code) for Peristeen. However, Coloplast will be applying for a national reimbursement code but the process of getting a specific code is a slow one. Until a code is granted, all selected patients will have to apply for reimbursement via a prior authorization process utilizing a miscellaneous code.
Medicare and other Federally funded health plans such as Tricare, will not cover Peristeen until there is an established national reimbursement code. Therefore, only patients with private or Medicaid insurance (in certain states) are able to apply for getting reimbursement at this time.
PCG Medical is the first provider to submit a prior authorization to Louisiana Medicaid. We are keeping our fingers crossed for Louisiana Medicaid to approve Peristeen for our patients and should know something soon!
To find out if you are a candidate for Peristeen & learn more please contact us at PCG Medical at support@pcgmed.com or 504-836-3899.








 Follow
Follow Forward
Forward